Full Length MockTest (PCM)
Test Instructions:
- This Mock test contains 75 MCQ questions.
- Choice and sequence for attempting questions will be as per the convenience of the candidate.
- Read each question carefully.
- Determine the one correct answer out of the four available options given for each question.
- Each question awards you 1 point for Chemistry , Physics 2 points for Maths.
- You can end the test any time by clicking “quiz summary>finish quiz”.
- The mock test will automatically be submitted when the time is up.
- You can review all questions once again and evaluate your mistakes by clicking “view questions” when the result is displayed.
- You can apply for Leaderboard when the result is displayed.
- You can re-attempt the exam as per your wish, yet scores of the first attempt will be recorded!
Test Summary
0 of 75 Questions completed
Questions:
Information
|
You must fill out this field. |
You have already completed the test before. Hence you can not start it again.
Test is loading…
You must sign in or sign up to start the test.
You must first complete the following:
Results
Results
0 of 75 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 75
1. Question
The angular velocity of a particle rotating in a circular orbit 100 times per minute is
CorrectIncorrect -
Question 2 of 75
2. Question
A mass m is supported by a massless string wound around a uniform hollow cylinder of mass m and radius R. If the string does not slip on the cylinder, with what acceleration will the mass fall on release?
CorrectIncorrect -
Question 3 of 75
3. Question
A bend in level road has a radius of 100 m. The maximum speed with which a car turning this bend without skidding, if coefficient of friction between the tyres and the surface of the road is 0.8, will be (g=9.8 m/s2)
CorrectIncorrect -
Question 4 of 75
4. Question
A swimmer while jumping into water from a height easily forms a loop in air, if
CorrectIncorrect -
Question 5 of 75
5. Question
A uniform horizontal circular platform of mass 200kg is rotating in 10 r.p.m. about a vertical axis passing through its centre. A boy of mass 500 Kg is standing on its edge. If the boy moves to the centre of the platform, then the frequency of rotation would be
CorrectIncorrect -
Question 6 of 75
6. Question
In the expression for Boyle’s laws , the product ‘PV’ has dimensions of
CorrectIncorrect -
Question 7 of 75
7. Question
For a gas R/ Cv =0.4, where ‘R’ is the universal gas constant and ‘Cv’ is molar specific heat at constant volume. The gas is made up of molecules which are
CorrectIncorrect -
Question 8 of 75
8. Question
Heat energy is incident on the surface at the rate of 100 J/min. If coefficient of absorption is 0.8 and coefficient of reflection is 0.1 then heat energy transmitted by the surface in 5 minute is
CorrectIncorrect -
Question 9 of 75
9. Question
An iron nail changes its colour from red to orange red and then to bluish white, when heated strongly in flames. This change of colour can be explained on the basis of
CorrectIncorrect -
Question 10 of 75
10. Question
An office room contains about 2000 moles of air. The change in the internal energy of this much air when it is called from 34℃ to 24ºC at a constant pressure of 1.0 atm is (Use ♈︎air = 1.4 and Universal gas constant = 8.314 J/mol K)
CorrectIncorrect -
Question 11 of 75
11. Question
 CorrectIncorrect
CorrectIncorrect -
Question 12 of 75
12. Question
 CorrectIncorrect
CorrectIncorrect -
Question 13 of 75
13. Question
 CorrectIncorrect
CorrectIncorrect -
Question 14 of 75
14. Question
 CorrectIncorrect
CorrectIncorrect -
Question 15 of 75
15. Question
 CorrectIncorrect
CorrectIncorrect -
Question 16 of 75
16. Question
 CorrectIncorrect
CorrectIncorrect -
Question 17 of 75
17. Question
Calculate the M.I. of a thin uniform ring about an axis tangent to the ring and in a plane of the ring, if its M.I. about an axis passing through the centre and perpendicular to plane is 4 kg m2
CorrectIncorrect -
Question 18 of 75
18. Question
The M.I. of disc about an axis perpendicular to its plane and passing through its centre is
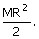 Its M.I. about a tangent perpendicular to its plane will be CorrectIncorrect
Its M.I. about a tangent perpendicular to its plane will be CorrectIncorrect -
Question 19 of 75
19. Question
Two bodies have their moments of inertia I and 2I respectively about their axis of rotation. If their kinetic energies of rotation are equal, their angular momenta will be in the ratio of
CorrectIncorrect -
Question 20 of 75
20. Question
One mole of ideal gas required 207 J heat to rise the temperature by 10°K when heated at constant pressure. If the same gas is heated at constant volume to raise the temperature by the same 10°K the heat required is (R = 8/3 J/mole °K)
CorrectIncorrect -
Question 21 of 75
21. Question
Mass of gas is 300 gm and its specific heat at constant volume is 750J/kg K. if gas is heated through 75°C at constant pressure of 105 N/m2, it expands by volume 0.08 × 106 cm3. find CP/CV
CorrectIncorrect -
Question 22 of 75
22. Question
The r.m.s. speed of the molecules of a gas in a vessel is 200 m/s. if 25% of the gas leaks out of the vessel, at constant temperature, then the r.m.s. speed of the remaining molecules will be
CorrectIncorrect -
Question 23 of 75
23. Question
The resistances in left and right gap of a meter bridge are 20 Ω and 30 Ω respectively. When the resistance in the left gap is reduced to half its value, the balance point shifts by
CorrectIncorrect -
Question 24 of 75
24. Question
In an LCR circuit as shown below both switches are open initially. Now switch S1 is closed, S2 kept open (q is charge on the capacitor and τ = RC is Capacitive time constant). Which of the following statement is correct?
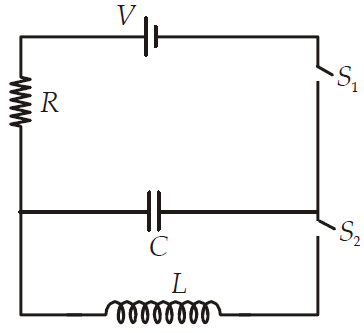 CorrectIncorrect
CorrectIncorrect -
Question 25 of 75
25. Question
Three resistances 2Ω, 3Ω and 4Ω are connected in parallel. The ratio of currents passing through them when a potential difference is applied across its ends will be
CorrectIncorrect -
Question 26 of 75
26. Question
Which type of ‘defect’ has the presence of cations in the interstitial sites?
CorrectIncorrect -
Question 27 of 75
27. Question
A crystalline solid XY3 has ccp arrangement for its element Y. X occupies_____.
CorrectIncorrect -
Question 28 of 75
28. Question
Select a ferromagnetic material from the following.
CorrectIncorrect -
Question 29 of 75
29. Question
In face-centred cubic unit cell, what is the volume occupied?
CorrectIncorrect -
Question 30 of 75
30. Question
Lithium has a bcc structure. Its density is 530 kg m-3 and its atomic mass is 6.94g mol-1. Calculate the edge length of a unit cell of lithium metal. (No = 6.02 ✕ 10⌃23 mol-1)
CorrectIncorrect -
Question 31 of 75
31. Question
The packing efficiency in simple public unit cell is ______ .
CorrectIncorrect -
Question 32 of 75
32. Question
A corner particle contributes its _______ part to the given unit cell.
CorrectIncorrect -
Question 33 of 75
33. Question
Which of the following in a hydrogen bonded molecular solid?
CorrectIncorrect -
Question 34 of 75
34. Question
Which of the following is a weak electrolyte ?
CorrectIncorrect -
Question 35 of 75
35. Question
Which one of the following pairs of solution is NOT an acidic buffer?
CorrectIncorrect -
Question 36 of 75
36. Question
Which of the following salt will give the highest pH in water?
CorrectIncorrect -
Question 37 of 75
37. Question
The pH of 10-4 M KOH solution will be _______.
CorrectIncorrect -
Question 38 of 75
38. Question
Which of the following CANNOT act both as Bronsted acid and as Bronsted base?
CorrectIncorrect -
Question 39 of 75
39. Question
Which of the following forms a basic buffer solution?
CorrectIncorrect -
Question 40 of 75
40. Question
What is the pH of millimolar solution of ammonium hydroxide which is 20% dissociated?
CorrectIncorrect -
Question 41 of 75
41. Question
The ratio of pH of 0.05 M and 0.005 M H2SO4 solutions will be _____.
CorrectIncorrect -
Question 42 of 75
42. Question
For the reversible reaction,
A(s) + B(g)
 C(g) + D(g) ΔG° = -350kJ,
C(g) + D(g) ΔG° = -350kJ,which one of the following statements is true? [Karnataka CET 2011]
CorrectIncorrect -
Question 43 of 75
43. Question
The enthalpy of vaporization of benzene is +35.3 kJ/mol at its boiling point of 80°C.The entropy change in the transition of vapour to liquid at its boiling point is ______. (in J mol−1 K−1)
CorrectIncorrect -
Question 44 of 75
44. Question
The pH of 0.01 M aqueous NaOH solution will be _______.
CorrectIncorrect -
Question 45 of 75
45. Question
A vessel at 1000 K contains CO2 with a pressure of 0.5 atm. Some of the CO2 is converted into CO on the addition of graphite. If the total pressure at equilibrium is 0.8 atm, the value of K is
CorrectIncorrect -
Question 46 of 75
46. Question
The number of atoms in 2.4 g of body-centred cubic crystal with edge length 200 pm is _____. (density = 10 g cm−3 , NA = 6 x 1023 atoms/mol)
CorrectIncorrect -
Question 47 of 75
47. Question
Volume occupied by single CsCl ion pair in a crystal is 7.014 x 10-23 cm3. The smallest Cs – Cs internuclear distance is equal to length of the side of the cube corresponding to volume of one CsCl ion pair. The smallest Cs to Cs internuclear distance is nearly _____.
CorrectIncorrect -
Question 48 of 75
48. Question
Statement 1: NaNO3 and CaO3 are isomorphous pairs.
Statement 2 : They have the same atomic ratios of the constituent atoms.
Statement 3 : They have different crystal structures.
Select the appropriate option.
CorrectIncorrect -
Question 49 of 75
49. Question
The coordination number of each sphere in a close packed one dimensional structure is ______.
CorrectIncorrect -
Question 50 of 75
50. Question
Silicon is a _____ as it conducts electricity better than _____but not as efficient as ________.
CorrectIncorrect -
Question 51 of 75
51. Question
 CorrectIncorrect
CorrectIncorrect -
Question 52 of 75
52. Question
 CorrectIncorrect
CorrectIncorrect -
Question 53 of 75
53. Question
 CorrectIncorrect
CorrectIncorrect -
Question 54 of 75
54. Question
 CorrectIncorrect
CorrectIncorrect -
Question 55 of 75
55. Question
 CorrectIncorrect
CorrectIncorrect -
Question 56 of 75
56. Question
 CorrectIncorrect
CorrectIncorrect -
Question 57 of 75
57. Question
 CorrectIncorrect
CorrectIncorrect -
Question 58 of 75
58. Question
 CorrectIncorrect
CorrectIncorrect -
Question 59 of 75
59. Question
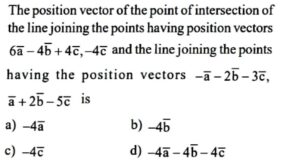 CorrectIncorrect
CorrectIncorrect -
Question 60 of 75
60. Question
 CorrectIncorrect
CorrectIncorrect -
Question 61 of 75
61. Question
 CorrectIncorrect
CorrectIncorrect -
Question 62 of 75
62. Question
 CorrectIncorrect
CorrectIncorrect -
Question 63 of 75
63. Question
 CorrectIncorrect
CorrectIncorrect -
Question 64 of 75
64. Question
 CorrectIncorrect
CorrectIncorrect -
Question 65 of 75
65. Question
 CorrectIncorrect
CorrectIncorrect -
Question 66 of 75
66. Question
 CorrectIncorrect
CorrectIncorrect -
Question 67 of 75
67. Question
 CorrectIncorrect
CorrectIncorrect -
Question 68 of 75
68. Question
 CorrectIncorrect
CorrectIncorrect -
Question 69 of 75
69. Question
 CorrectIncorrect
CorrectIncorrect -
Question 70 of 75
70. Question
 CorrectIncorrect
CorrectIncorrect -
Question 71 of 75
71. Question
 CorrectIncorrect
CorrectIncorrect -
Question 72 of 75
72. Question
 CorrectIncorrect
CorrectIncorrect -
Question 73 of 75
73. Question
 CorrectIncorrect
CorrectIncorrect -
Question 74 of 75
74. Question
 CorrectIncorrect
CorrectIncorrect -
Question 75 of 75
75. Question
 CorrectIncorrect
CorrectIncorrect
